* The activities of the Informal Regulatory Guidance on Medical Devices were launched in 2023.
The number of scientific advice cases in 2018-2023 in an accessible format (pdf).
Supporting innovation and development of infrastructure

Challenges
The development of medicines and medical devices is an industry that requires modern technology and a high level of education. In addition to the pharmaceutical industry, high-quality scientific research is promoted in Finland, for example in university hospitals and universities. Both resources and infrastructure that supports research are needed for these operations. As a country with a small population and thus a small market area, Finland strives to participate actively in the European Medicines Agency network to ensure the availability of medicines and medicinal products in Finland.
Supporting innovations aimed at the research, manufacture and use of medicines and medical devices is also important for economic and ecological sustainability. Supporting innovations will ensure the cost-effectiveness of healthcare, investments in the pharmaceutical sector and the employment of experts in Finland also in the future. The development of new medicines and medical devices can facilitate the production of more environmentally friendly and efficient products and devices.
In Finland, all clinical trials on medicinal products and devices undergo a scientific and ethical ex-ante evaluation, which enables Finns to access the latest research treatments. Uniform data resources are also important to ensure the safe and efficient use of medicines. Data resources enable different actors and citizens to quickly find reliable information on medicines and their use.
UN Sustainable Development Goal 9:

9.1 Develop high-quality, reliable and sustainable infrastructure, such as regional and cross-border infrastructure, to support economic development and people’s well-being by investing in affordable and equal access for all.
Fimea’s role and objective
Ensuring and developing a high-quality and safe pharmaceutical sector plays a central role in Fimea’s operations. Fimea’s objective from the perspective of infrastructure includes:
- Develop pharmaceutical sector infrastructure (information systems, service systems, and pharmacy systems) with supporting studies, surveys and development projects, both independently and with stakeholders
- Carrying out research, analysis and development work related to the pharmacy economy and the development of the distribution of medicines
- Developing the pharmaceutical sector, e.g. through the drafting of legislation and informational guidance
- Acts as a representative in the Pharmacy and Financing Division of the Ministry of Social Affairs and Health and produces information as a basis for pharmacotherapy guidance.
How the successful attainment of the objective is promoted
Fimea promotes the aforementioned objectives by diversely participating in projects that will develop the pharmaceutical sector and by offering open information products for citizens and health care professionals. Examples of Fimea's 2023 development projects listed below:
- Fimea carried out the preparation of the medicines data repository in cooperation with key actors, commissioned by the Ministry of Social Affairs and Health. When implemented, the medicines data repository will bring together material related to the tasks and services of different authorities on pharmaceutical substances and products as well as on the pharmaceutical market and consumption. The aim is to provide the collected data for use as information products, information system services and user interfaces.
- Fimea began the reform of the pharmacy register by compiling information on the retail distribution of medicines (pharmacies, secondary pharmacies, pharmacy service points and distance selling services as well as hospital pharmacies and pharmaceutical centres) in a shared and maintained database. The reform will enable the availability of up-to-date and accessible information, the further development of electronic services for pharmacy operations and smoother information cooperation with Kela and Finnish Institute for Health and Welfare (THL).
- Fimea and Kela worked together to develop antimicrobial reporting, which describes the consumption of systemic antibacterial, antifungal and antiviral medicines in Finland. This development project resulted in a dynamic public report containing consumption data at the national and wellbeing services county level. In addition, a report intended for official users containing more detailed consumption data at the national, wellbeing services county and municipal level will be published.
- Fimea participated in the UNICOM project, which focuses on improving patient safety and enabling better healthcare for all. Together with Kela, Fimea will focus on the implementation of the International Standardisation Organisation's (ISO) Identification of Medicinal Products (IDMP) standard in Finland, which will promote the accurate identification of medicines globally and enable cross-border prescriptions.
- In 2023, Fimea piloted the application procedure for the HTA assessment of hospital medicines. The aim of the reform is to harmonise the assessments related to the introduction of outpatient and hospital medicines. The purpose of the reform is also to improve the coverage, predictability and timeliness of assessments.
What does HTA mean?
The term HTA (Health Technology Assessment) refers to the assessment of health care methods. At Fimea, HTA assessments are chiefly carried out on new hospital medicines that have been granted marketing authorisation when it is necessary to assess their therapeutic effects, cost-effectiveness and budget impact based on research evidence. A new medicine is compared with other treatment options for the same disease.
Fimea's HTA assessment can be initiated either on the initiative of a pharmaceutical company or Fimea, and it is based on publicly available information and information provided by a pharmaceutical company. A specialist, who is familiar with the illness in question and its treatment, also takes part in HTA assessments. The chief purpose of HTA assessments is to inform the Council for Choices in Health Care in Finland (COHERE Finland), which provides a national recommendation on the use of the assessed medicine in hospitals. More detailed information on Fimea's HTA assessment process can be found in the animation published by Fimea on YouTube.
- Fimea, HUS, the wellbeing services counties of Pirkanmaa and Southwest Finland and Sitra carried out a cooperation pilot project aimed at testing an operating model that would enable the production of additional evidence on new pharmacotherapies. In the pilot, patient data was extracted from the OMOP databases of university hospitals in Tampere, Turku and Helsinki, and the functionality of data production was tested from the perspective of the information needs of HTA activities. The pilot promoted cooperation between Fimea, wellbeing services counties and the FinOMOP consortium and clarified the roles and responsibilities related to national healthcare and social welfare data production.
UN Sustainable Development Goal 9:

9.5 Increase scientific research, update technological capabilities of industries in all countries, especially in developing countries, for example by encouraging innovation and significantly increasing the number of R&D staff per million inhabitants and public and private funding for R&D by 2030.
Fimea’s role and objective
Fimea’s objective is to increase scientific research in the pharmaceutical sector, provide advice and support for operators in their development projects and encourage innovation. Fimea’s objectives from the perspective of innovation include:
- Participation in responsible duties in the assessment of marketing authorisation applications for medicines and clinical trial applications and in the inspections of pharmaceutical operators.
- Contribute to the preconditions for innovation activities in the pharmaceutical sector and to obtaining foreign research investments in Finnish infrastructure.
- Provide scientific advice and guidance to operators in the pharmaceutical sector:
- Increase medicines and device research by guiding and advising researchers on their projects.
- Provide guidance and communication, training and support in official processes for sponsors of clinical trials on medicinal products and medical devices, including non-commercial research groups.
- Support business activities with means such as guiding medicines manufacturing and distribution, the biosector and medicinal products.
How the successful attainment of the objective is promoted
- Fimea provides support for the development of medicines and medical devices in order to promote innovation activities in both academia and business life. There are many types of advisory services available at both the national and international level:
- Fimea offers free and informal advisory services to pharmaceutical developers through the Medication counselling clinic, which is specifically intended for early-stage innovators of pharmaceutical development. For companies that have progressed slightly further in their research, Fimea offers fee-based scientific advice.
- In the spring of 2023, Guidance on Medical Devices launched its activities. The Guidance service offers researchers tailored assistance to meet official requirements.
- In 2023, Fimea assessed seven studies on the combined use of medicines and devices. Six of these were performance studies and one was a study in which the device administered the medicine the study concerned. In a webinar held in May 2023, stakeholders were provided training on the special features of combination trials.
- Fimea provides advice through the EMA on medicines development projects to international research groups and companies.
- Fimea also participates through the EMA in the Quality Innovation Group to support production and analysis methods for innovative medicines and in the Innovation Task Force to support individual development projects.
Scientific advisory service for pharmaceutical companies
The efficacy and safety of medicines must be studied reliably, and for this reason, the authorities provide pharmaceutical companies instructions and advice at any stage of pharmaceutical development. The Committee for Medicinal Products for Human Use (CHMP) under the European Medicines Agency (EMA) provides scientific advice to support pharmaceutical development at the recommendation of the Scientific Advice Working Party (SAWP) or the Emergency Task Force (ETF). Fimea may also provide scientific advice as national activities or advise companies as low-threshold Medication counselling clinic activities.
In EMA's scientific advisory service, the body developing the medicine sends its questions related to the development of medicines to the EMA, from where the question is forwarded to experts in two Member States. The response time is approximately four weeks, after which a working group discusses the draft responses and combines them into one comprehensive response. If the Member States disagree in their responses, the working group will decide on a common position. The CHMP approves the final answer. The EMA Scientific Advisory Group has representatives from different areas of expertise (quality, clinical and non-clinical issues and methodology). The Group includes five experts from Finland. For more information on the EMA's advisory services please see the EMA website.
| Type of advice Sort the table ascending by the column | 2018 Sort the table ascending by the column | 2019 Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column |
|---|---|---|---|---|---|---|
| EMA/SAWP advice | 67 | 78 | 96 | 126 | 103 | 86 |
| National scientific advice (human medicines) | 10 | 13 | 11 | 8 | 8 | 5 |
| Medication counselling clinic (human medicines) | 9 | 8 | 8 | 0 | 0 | 6 |
| Medical Device Advisory Clinic* | - | - | - | - | - | 9 |
| National scientific advice (veterinary medicines) | 0 | 0 | 0 | 0 | 1 | 0 |
| Medication counselling clinic (veterinary medicines) | 2 | 1 | 1 | 1 | 0 | 0 |
- The number of marketing authorisation assessments for medicines carried out by Fimea as the responsible rapporteur or co-rapporteur country for the EMA-coordinated centralised marketing authorisations is high compared to the size of the agency.
| Marketing authorisation transactions Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column |
|---|---|---|---|---|
| Centralised procedure reporting tasks | 785 | 841 | 920 | 895 |
| Centralised procedure parallel reporting tasks | 1 323 | 875 | 960 | 297 |
There is a clear change in the number of parallel reporting tasks in 2023 compared to previous years, because the number of applications entered into Fimea's marketing authorization system has been reduced during 2023. Omitted entry of the following parallel reporting tasks: type II (except indication extensions), type I variations, notifications and obligations.
The number of marketing authorisation transactions in 2020-2023 in an accessible format (pdf).
- Since February 2023, only EU Regulation 536/2014 has been applied in the assessment of new clinical trials. The aim is to increase the EU's competitiveness as an area where clinical trials are performed, harmonise operating methods in different Member States and streamline the market authorisation procedure. The joint safety assessment under the same regulation will continue with the support of the Safe-CT programme under the EU4Health project. In 2023, Fimea:
- started as the responsible rapporteur country in 24 multinational research applications and assessed a total of 108 new research applications and notifications.
- was responsible for the safety reporting of 23 pharmaceutical substances.
- assessed a total of 494 notifications of changes in clinical trials in accordance with directive legislation during the transition period.
* Includes both notifications and applications for authorisation.
Number of notifications of clinical trials in 2018–2023 in an accessible format (pdf).
- Fimea strives to meet the challenges posed by the continuous evolution of artificial intelligence and growing datasets by advocating at the national and EU level and by developing personnel competence.
- In 2023, a representative of Fimea joined the HMA-EMA Big Data Steering Group. The steering group strives to increase the benefits gained from data in pharmaceutical regulation, and its vision is to integrate data analysis into the regulatory evaluation processes and thus improve the quality of decision-making. The ultimate objective is to act in the interests of patients and to improve both human and animal health in the EU through better medicines regulation.
- Fimea's internal RWD/AI/ML network aims to promote the systematic sharing of topical issues and competence related to real-world data (RWD), artificial intelligence (AI) and machine learning (ML). In addition, the network aims to influence RWD issues both nationally and in the EU and to identify the opportunities and risks related to AI/ML topics from the perspectives of the work of authorities as well as pharmaceutical research and pharmacotherapy. In 2023, Fimea organised four RWD/AI/ML network forums and published nine news summaries.
- The aim of Fimea's research activities is to promote the rational use of medicines and support societal decision-making to promote public health and increase the wellbeing of the population.
- Fimea coordinated the Research Network for Rational Pharmacotherapy (RATTI), which organised a discussion event in 2023 where challenges related to rational pharmacotherapy were identified through an expert lecture and panel discussion. The RATTI Network also publishes Policy Briefs (summaries) by research groups, which present the research results of rational pharmacotherapy researchers. Policy Briefs are intended to support decision-making. A total of twelve Policy Briefs and press releases were published by research teams on the start of studies.
- Fimea's researchers actively participate in versatile research projects. The results of these were published in 2023 in a total of 16 original publications and one research publication in the publication series Fimea kehittää, arvioi ja informoi (Fimea develops, assesses and informs). The topics of the 2023 publications included:
- Use of medicines by the elderly
- Impacts of the Covid-19 pandemic on the use of antibiotics
- Implementation of medicines advice in pharmacy distance selling services
- Experiences of citizens related to medicines, pharmacotherapy management and advice
- Regulation of academic medicines development and related advice in EU countries
- Implementation of a cross-border electronic prescription
- Statements issued by patient organisations concerning decisions on the substitutability and introduction of medicines
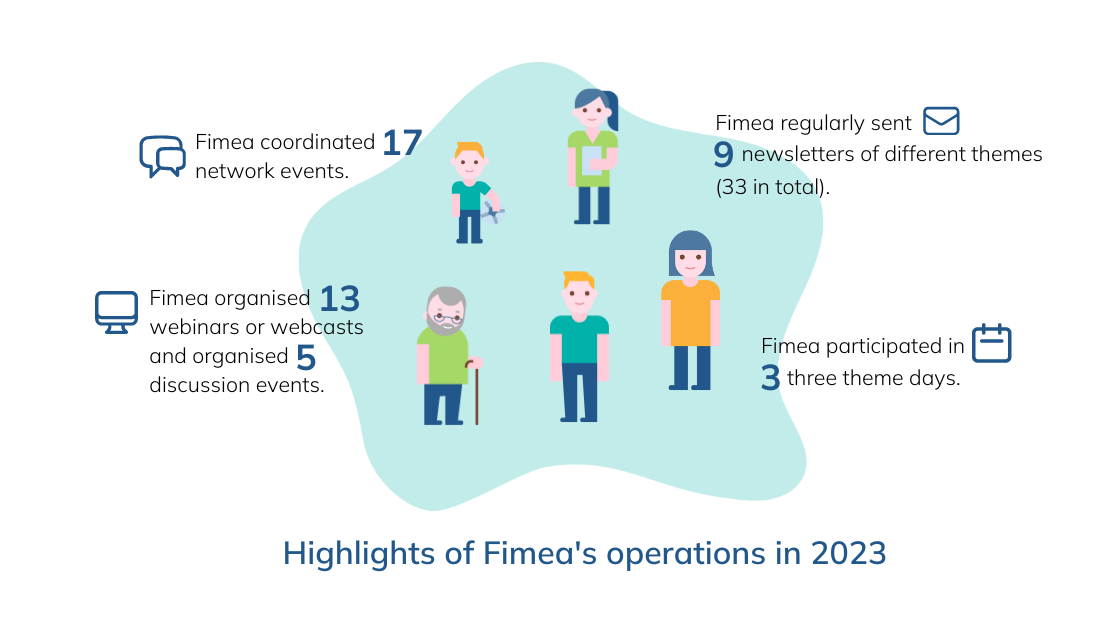
Highlights of Fimea's operations in 2023 (the image in an accessible format, pdf).